You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
soak ?
- Thread starter victoroni
- Start date
Dennis Morland
KNIFE MAKER
Once your knife blank reaches a desired temperature, you let it set (soak) at that temperature for a certain period of time.
Dennis Morland
KNIFE MAKER
I am glad I could help you out. My friends call me DeMo. Dennis is fine, also. Mr. Morland is way too formal.
EdCaffreyMS
"The Montana Bladesmith"
I'm gona give a warning...and climb up on my soapbox.....
I don't know who, or how things got so sideways when it comes to "soaking" during heat treat. There are SOME steels that require soaking,due to their wide range of element content, and that soaking is necessary to equally distribute the elements throughout the piece of steel....also called "in solution" in heat treating terms.
HOWEVER, you read all the time on the net about people recommending extended soaks (meaning WAY beyond what's necessary) on SIMPLE steels like the 10XX series..... which are steel that contain ONLY Carbon, iron, and manganese. Extended soak time on those steels where it IS NOT NECESSARY, ONLY DEGRADES THE STEEL, AND GIVE YOU ENORMOUS GRAIN SIZE IN THE FINISHED PIECE! Simple carbon steels, as well as many of the lower alloy steels such as 5160, O1, and similar requires, and in fact it's far better overall for the steel and grain size....to just bring them up to the specified austinizing temp (hardening temp), with minimal or now soak, and get them into the quench, rather then to over do a soak.
Here's a tip..... whenever you perform ANY given step/process that involves heat treatment..... and a given temp is specified.... the steel MUST BE AT THE SPECIFIED TEMP WHEN THE ACTION OCCURS! What does that mean? Let's use hardening/quenching as an example....if the specified austinizing (hardening) temp for the given steel is 1500F (just for example)..... that means the steel must be NO LOWER THEN 1500F WHEN IT HITS THE QUENCH OIL/QUENCHANT. Personally I think all these outlandish soak times have come from people who have failed to do that, then because they kept upping and upping their soak times, they finally managed to have the steel at the correct temp when it hit the quench......so now they believe that everything must have the daylights soaked out of it (read that as way more then needed) prior to quenching. Problem is, I've seen, handled, and tried to cut with many blades that people have soaked for extended times....and frankly the blades are crap in every aspect that matters for a knife..... they don't cut well at all, the edges usually chip badly, and most shatter with as little as 15-20 degrees deflection. So what's the take away? Simply this.....DO NOT BELIEVE WHAT YOU READ ON THE NET ABOUT EXTENDED SOAKING STEEL/BLADES, ESPECIALLY SIMPLE CARBON AND SIMPLE ALLOY STEELS! These steels are best when brought up to the specified temp, and at long as they are AT THE GIVEN temp when they hit the quench....they will be FAR better blades then those that have soaked for too long!
I don't know who, or how things got so sideways when it comes to "soaking" during heat treat. There are SOME steels that require soaking,due to their wide range of element content, and that soaking is necessary to equally distribute the elements throughout the piece of steel....also called "in solution" in heat treating terms.
HOWEVER, you read all the time on the net about people recommending extended soaks (meaning WAY beyond what's necessary) on SIMPLE steels like the 10XX series..... which are steel that contain ONLY Carbon, iron, and manganese. Extended soak time on those steels where it IS NOT NECESSARY, ONLY DEGRADES THE STEEL, AND GIVE YOU ENORMOUS GRAIN SIZE IN THE FINISHED PIECE! Simple carbon steels, as well as many of the lower alloy steels such as 5160, O1, and similar requires, and in fact it's far better overall for the steel and grain size....to just bring them up to the specified austinizing temp (hardening temp), with minimal or now soak, and get them into the quench, rather then to over do a soak.
Here's a tip..... whenever you perform ANY given step/process that involves heat treatment..... and a given temp is specified.... the steel MUST BE AT THE SPECIFIED TEMP WHEN THE ACTION OCCURS! What does that mean? Let's use hardening/quenching as an example....if the specified austinizing (hardening) temp for the given steel is 1500F (just for example)..... that means the steel must be NO LOWER THEN 1500F WHEN IT HITS THE QUENCH OIL/QUENCHANT. Personally I think all these outlandish soak times have come from people who have failed to do that, then because they kept upping and upping their soak times, they finally managed to have the steel at the correct temp when it hit the quench......so now they believe that everything must have the daylights soaked out of it (read that as way more then needed) prior to quenching. Problem is, I've seen, handled, and tried to cut with many blades that people have soaked for extended times....and frankly the blades are crap in every aspect that matters for a knife..... they don't cut well at all, the edges usually chip badly, and most shatter with as little as 15-20 degrees deflection. So what's the take away? Simply this.....DO NOT BELIEVE WHAT YOU READ ON THE NET ABOUT EXTENDED SOAKING STEEL/BLADES, ESPECIALLY SIMPLE CARBON AND SIMPLE ALLOY STEELS! These steels are best when brought up to the specified temp, and at long as they are AT THE GIVEN temp when they hit the quench....they will be FAR better blades then those that have soaked for too long!
Last edited:
Chris Railey
Well-Known Member
Yep...Ed is feeling better. Good for you sir.I'm gona give a warning...and climb up on my soapbox.....
I don't know who, or how things got so sideways when it comes to "soaking" during heat treat. There are SOME steels that require soaking,due to their wide range of element content, and that soaking is necessary to equally distribute the elements throughout the piece of steel....also called "in solution" in heat treating terms.
HOWEVER, you read all the time on the net about people recommending extended soaks on SIMPLE steels like the 10XX series..... which are steel that contain ONLY Carbon, iron, and manganese. Extended soak time on those steels where it IS NOT NECESSARY, ONLY DEGRADES THE STEEL, AND GIVE YOU ENORMOUS GRAIN SIZE IN THE FINISHED PIECE! Simple carbon steels, as well as many of the lower alloy steels such as 5160, O1, and similar requires, and in fact it's far better overall for the steel and grain size....to just bring them up to the specified austinizing temp (hardening temp) and get them into the quench.
Here's a tip..... whenever you perform ANY given step/process that involves heat treatment..... and a given temp is specified.... the steel MUST BE AT THE SPECIFIED TEMP WHEN THE ACTION OCCURS! What does that mean? Let's use hardening/quenching as an example....if the specified austinizing (hardening) temp for the given steel is 1550F..... that means the steel must be NO LOWER THEN 1550F WHEN IT HIT THE QUENCH OIL/QUENCHANT. Personally I think all these outlandish soak times have come from people who have failed to do that, then because they kept upping and upping their soak times, they finally managed to have the steel at the correct temp when it hit the quench......so now they believe that everything must have the crap soaked out of it prior to quenching. Problem is, I've seen handle, and tried to cut with many blades that people have soaked for extended times....and frankly the blades are crap in every aspect that matters for a knife..... they don't cut well at all, the edges usually chip badly, and most shatter with as little as 15-20 degrees deflection. So what's the take away? Simply this.....DO NOT BELIEVE WHAT YOU READ ON THE NET ABOUT SOAKING STEEL/BLADES, ESPECIALLY SIMPLE CARBON AND SIMPLE ALLOY STEELS! These steels are best when brought up to the given temp, and at long as they are AT THE GIVEN temp when they hit the quench....they will be FAR better blades then those that have soaked for too long!
OK, I'll stop now.....I'm am just so sick and tired of someone who has made 5 knives, telling me, that they "soak 1095 for 30 mins. and it makes a great knife." Sorry kid.....I've been at this for 30+ years, and if you wanna argue about it, put your money where your mouth is, and lets have a head to head cutting/torture test and see who's blade survives......I can promise that it ain't gona be one that has soaked too long.
EdCaffreyMS
"The Montana Bladesmith"
Sorry.... I was feeling spunky there for a minute! 
Kevin R. Cashen
Super Moderator
I actually thought that I should say something earlier in this thread in hopes of heading off any misunderstandings. I have never really taken a firmer stance about this topic because it’s just a small point about steel heat treatment. But every time this comes up things get more and more confused.
There are not just certain steels that require soaking, but the vast majority of steels, the simple carbon steels are actually the small exception to this. I have done a lot of studying of overheated 10XX and I am not seen too many internal processes that could degrade the steel that much. Short of burning, the most you will get is heavier decarb and, indeed, some grain growth, but to say enormous grain size seems extreme compared with my experience of the effects of soak time alone.
But what prompts me to write is to also point out that some alloy steels like 5160, and especially O-1, which I have been working with my entire career, benefit from a soak. I have decades of well documented research to show that just bringing O-1 to temperature and quenching, with no soak, is wasting that steel when you could indeed just use 1084.
I am also sorry, but I'm going to get a little techy here. Ac1 and Ar1 are two different temperatures and the effects of hysteresis on diffusional processes are profound. I teach and demonstrate the wide temperature differences between decalescence and recalescence regularly to students in my shop and across the country. And while it is always best to get into the oil as soon as possible there as a huge space between 1500°F and recalescence. I can assure you that it is not necessary for a steel to be exactly at the austenitizing temperature when it hits the oil, it just needs to be safely above Ar1 (recalescence) and the temp necessary to keep your desired level of proeutectoid phases in solution. *
And it is temperature that is really at the heart of this issue, rather than time. None of the steels mentioned so far should ever see more than 1500°F, with the sole exception of 5160 at 1525°F. It is overheating that results in grain growth. A modern fine-grained deoxidized steel, due to its chemistry, will have a grain coarsening temperature that, once exceed, will display rapid grain growth, regardless of the time at temperature, but certainly with increased times. Any high carbon 10XX steel heated to 1500°F and beyond will tolerate no soaking whatsoever because it has already been overheated.
But I am not sure how much we may actually disagree, because the goal posts always seem to be shifting. For the most part I am getting a clear message that any soaking whatsoever could be disastrous. But that often morphs into truly long soaks in the range of 30 minutes or more, which I don’t think anybody would argue is excessive. But then no time, much as less 30 minutes, was mentioned in any other posts here. So, I may be misunderstanding you Ed, thus, help me better understand, are you saying that any soaking is detrimental to the steel, or only soaking for too long, like 30 minutes?
To all those participating in this thread, I am sorry if this time around seems more disjointed, and let me be clear that I actually hate having to get6 too techy in clearing things up, but this topic really needs to finally be cleared up for the good of those who we hope to help.
So, for anybody visiting this thread, what I would rather do is happily offer any information I can to answers any questions, in a friendly and informative conversation, on the soak times of various steels, and what goes on inside the steel when it happens. I would be especially happy to work through the specifics of Ed’s negative experiences with the process and see if we can get to the bottom of where things may have gone wrong to lead to such divergent observations in this area.
*I would particularly like the opportunity to explain this paragraph in English, due to the egghead jargon I was forced to use to fit it on the page.
There are not just certain steels that require soaking, but the vast majority of steels, the simple carbon steels are actually the small exception to this. I have done a lot of studying of overheated 10XX and I am not seen too many internal processes that could degrade the steel that much. Short of burning, the most you will get is heavier decarb and, indeed, some grain growth, but to say enormous grain size seems extreme compared with my experience of the effects of soak time alone.
But what prompts me to write is to also point out that some alloy steels like 5160, and especially O-1, which I have been working with my entire career, benefit from a soak. I have decades of well documented research to show that just bringing O-1 to temperature and quenching, with no soak, is wasting that steel when you could indeed just use 1084.
I am also sorry, but I'm going to get a little techy here. Ac1 and Ar1 are two different temperatures and the effects of hysteresis on diffusional processes are profound. I teach and demonstrate the wide temperature differences between decalescence and recalescence regularly to students in my shop and across the country. And while it is always best to get into the oil as soon as possible there as a huge space between 1500°F and recalescence. I can assure you that it is not necessary for a steel to be exactly at the austenitizing temperature when it hits the oil, it just needs to be safely above Ar1 (recalescence) and the temp necessary to keep your desired level of proeutectoid phases in solution. *
And it is temperature that is really at the heart of this issue, rather than time. None of the steels mentioned so far should ever see more than 1500°F, with the sole exception of 5160 at 1525°F. It is overheating that results in grain growth. A modern fine-grained deoxidized steel, due to its chemistry, will have a grain coarsening temperature that, once exceed, will display rapid grain growth, regardless of the time at temperature, but certainly with increased times. Any high carbon 10XX steel heated to 1500°F and beyond will tolerate no soaking whatsoever because it has already been overheated.
But I am not sure how much we may actually disagree, because the goal posts always seem to be shifting. For the most part I am getting a clear message that any soaking whatsoever could be disastrous. But that often morphs into truly long soaks in the range of 30 minutes or more, which I don’t think anybody would argue is excessive. But then no time, much as less 30 minutes, was mentioned in any other posts here. So, I may be misunderstanding you Ed, thus, help me better understand, are you saying that any soaking is detrimental to the steel, or only soaking for too long, like 30 minutes?
To all those participating in this thread, I am sorry if this time around seems more disjointed, and let me be clear that I actually hate having to get6 too techy in clearing things up, but this topic really needs to finally be cleared up for the good of those who we hope to help.
So, for anybody visiting this thread, what I would rather do is happily offer any information I can to answers any questions, in a friendly and informative conversation, on the soak times of various steels, and what goes on inside the steel when it happens. I would be especially happy to work through the specifics of Ed’s negative experiences with the process and see if we can get to the bottom of where things may have gone wrong to lead to such divergent observations in this area.
*I would particularly like the opportunity to explain this paragraph in English, due to the egghead jargon I was forced to use to fit it on the page.
Last edited:
Kevin R. Cashen
Super Moderator
To the original question:
Steel is an alloy, or even more simply, a mixture of iron and carbon. At room temperature the carbon will separate out to concentrate enough to form a compound (iron carbide) within the iron, leaving the iron free, and unfortified, to behave like the rather soft metal that it is. To harden the steel, we need to convert that iron carbide into free carbon that we can use to “fortify” the iron matrix and stiffen things up. If the carbides are fine, when we heat the steel to above 1335°F they will be quickly dissolved and the internal makeup of the steel will be converted to a solid solution of carbon in iron known as austenite. Thus, a simple carbon steel may not need any extended time above 1335°F to reach full solution, and the higher above that temperature you go, the less time it takes for full solution.
Two huge factors in, modern steel, affect this first paragraph. The size and distribution of those carbides, and alloying elements beyond carbon which can create much more complex carbides or slow down rates of carbon movement into the solution. If the carbides a very large and segregated, it will take more time, or greater temperature, to put them evenly into solution. But temperature is so powerful that it is coarse tuning, while time is better at fine tuning. Temperature is like using a sledge hammer to finishing an engraving job in a minute, while time is like using a little chasing hammer to do the engraving precisely in an hour. Blasting steel into solution with overheating will most likely lead to grain enlargement or oversaturating the solution, so a lower temperature with a little more time is more precise and safer.
So, if you have course carbides, if they are tougher carbides to break, or alloying will slow the rate of the carbon movement, soak times become important. As previously mentioned, the soak time starts when the steel reaches the target temperature, so if you put your steel in the oven set to 1475°F, and it drops to 1435°F, you need to wait for the oven to read 1475°F again to start the timer. Now there are no set times that are law here because it changes with each steel and the condition it is in from previous thermal operations.
For steels like 1075, 1080, 1084, 1095, W1, and W2 the time could be the moment you reach 1475°F again, as these are very simple iron carbides, but it could be a few minutes of you have a coarser carbide condition. In any case, more than 5 to 10 minutes would be extreme and counter-productive for these steels. For these steels the shorter the time to achieve even solution the better.
Next would come a steel with a smidge for chromium like 5160. Not only doe this steel have a larger metal alloy atom in the matrix it also has less than .77%-.80% carbon, which means it needs a little more temperature to even out the solution, so it is best austenitized at 1525°F, and will definitely achieve a more uniform solution with a little time at that temp. If the particles are fine, not much, if it is coarse, a few more minutes.
An alloyed tool steel like O-1 has a good helping of Chromium carbides and even some tungsten or vanadium. It absolutely needs some time to break the carbon free and spread it through the matrix. And if the carbide is coarse, it could take more time than you think. But more importantly all those additions to the chemistry puts the level of “too much time” way up there.
I work with simpler alloyed steels like O-1, L6, 52100 and some others, and my standard soak time is 10 minutes. I find that If I have to go much beyond that, it may be better to normalize the steel and come at it again for the carbides are probably too coarse. But if you are dealing with the steel as you receive it from your supplier, and you are not up to the ins and outs of normalizing, you may need to bump up your temp or extend your time a bit longer.
This is one thing that I have found for certain about O-1. In a spheroidized condition, almost certainly how it will come from the supplier, a less than proper soak will give the performance of something like 1075 or 1084 at best. But O-1 with a soak time that will allow it to achieve a decent solution will easily outpace those steels significantly, this could be from 10 to 15 minutes at 1475°F, depending upon the coarseness of the carbide.
Carbon diffusion always has two factors- temperature and time, more of one will equal less of the other, but the other is always there to some extent.
Steel is an alloy, or even more simply, a mixture of iron and carbon. At room temperature the carbon will separate out to concentrate enough to form a compound (iron carbide) within the iron, leaving the iron free, and unfortified, to behave like the rather soft metal that it is. To harden the steel, we need to convert that iron carbide into free carbon that we can use to “fortify” the iron matrix and stiffen things up. If the carbides are fine, when we heat the steel to above 1335°F they will be quickly dissolved and the internal makeup of the steel will be converted to a solid solution of carbon in iron known as austenite. Thus, a simple carbon steel may not need any extended time above 1335°F to reach full solution, and the higher above that temperature you go, the less time it takes for full solution.
Two huge factors in, modern steel, affect this first paragraph. The size and distribution of those carbides, and alloying elements beyond carbon which can create much more complex carbides or slow down rates of carbon movement into the solution. If the carbides a very large and segregated, it will take more time, or greater temperature, to put them evenly into solution. But temperature is so powerful that it is coarse tuning, while time is better at fine tuning. Temperature is like using a sledge hammer to finishing an engraving job in a minute, while time is like using a little chasing hammer to do the engraving precisely in an hour. Blasting steel into solution with overheating will most likely lead to grain enlargement or oversaturating the solution, so a lower temperature with a little more time is more precise and safer.
So, if you have course carbides, if they are tougher carbides to break, or alloying will slow the rate of the carbon movement, soak times become important. As previously mentioned, the soak time starts when the steel reaches the target temperature, so if you put your steel in the oven set to 1475°F, and it drops to 1435°F, you need to wait for the oven to read 1475°F again to start the timer. Now there are no set times that are law here because it changes with each steel and the condition it is in from previous thermal operations.
For steels like 1075, 1080, 1084, 1095, W1, and W2 the time could be the moment you reach 1475°F again, as these are very simple iron carbides, but it could be a few minutes of you have a coarser carbide condition. In any case, more than 5 to 10 minutes would be extreme and counter-productive for these steels. For these steels the shorter the time to achieve even solution the better.
Next would come a steel with a smidge for chromium like 5160. Not only doe this steel have a larger metal alloy atom in the matrix it also has less than .77%-.80% carbon, which means it needs a little more temperature to even out the solution, so it is best austenitized at 1525°F, and will definitely achieve a more uniform solution with a little time at that temp. If the particles are fine, not much, if it is coarse, a few more minutes.
An alloyed tool steel like O-1 has a good helping of Chromium carbides and even some tungsten or vanadium. It absolutely needs some time to break the carbon free and spread it through the matrix. And if the carbide is coarse, it could take more time than you think. But more importantly all those additions to the chemistry puts the level of “too much time” way up there.
I work with simpler alloyed steels like O-1, L6, 52100 and some others, and my standard soak time is 10 minutes. I find that If I have to go much beyond that, it may be better to normalize the steel and come at it again for the carbides are probably too coarse. But if you are dealing with the steel as you receive it from your supplier, and you are not up to the ins and outs of normalizing, you may need to bump up your temp or extend your time a bit longer.
This is one thing that I have found for certain about O-1. In a spheroidized condition, almost certainly how it will come from the supplier, a less than proper soak will give the performance of something like 1075 or 1084 at best. But O-1 with a soak time that will allow it to achieve a decent solution will easily outpace those steels significantly, this could be from 10 to 15 minutes at 1475°F, depending upon the coarseness of the carbide.
Carbon diffusion always has two factors- temperature and time, more of one will equal less of the other, but the other is always there to some extent.
Kevin R. Cashen
Super Moderator
And finally, before I go to bed as my insomnia has passed:
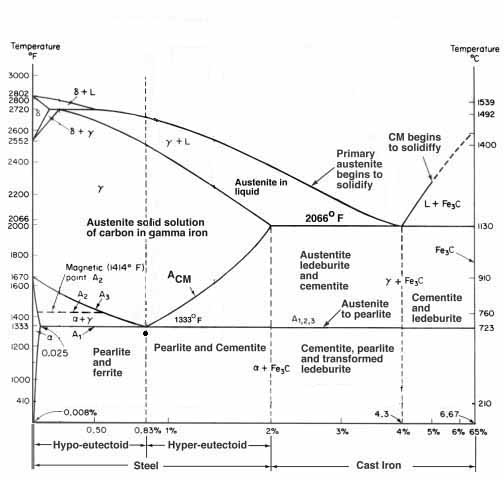
It is very important to note that this familiar chart is often referred to as the iron carbon equilibrium diagram. This is because the phases shown at the given temperatures are what is achieved at near equilibrium. In plainer English, the phases represented are what you will get if the steel is held at that temperature. It assumes some level of soaking. And the reason the “critical temperature” on heating (Ac1) is very different than the “critical temperature” on cooling (Ar1) is that rising or falling temperatures are no longer holding that equilibrium and pushes the temperatures all over the place. Things dissolve as much as 100°F higher than they come back out of solution, depending on how fast the heating or cooling is. To get them closer together, you need to hold for equilibrium conditions.
Folks can give this a try. Heat a piece of steel until you chase the shadow out of its glow and the magnet stops sticking, i.e. decalescence. Look at how bright this is and remember it. As it cools watch how dim, or dark, it is before you get the brighter energy wave that moves through it and the magnet starts to grab. The steel is almost black again. This is the difference heat and cooling, not holding for a time at a transformation temperature, makes. A1 is but one of four “critical temperatures” we typically deal with in heat treating a knife.

I have shared this image many times before across the internet, but I find that after all these years it is often still needed. This is the fractured end grain size of O-1 that was soaked for 5 hours at around ten degrees higher than its recommended range. I think we can take grain enlargement right off the table for things to worry about with soaking an alloy steel. I have often called grain growth the bladesmith’s bogeyman due to the greatly overexaggerated threat it poses.
What defines an austenite grain is the grain boundary. It is mostly the interface of two different crystalline stacking orientations, but its existence is also reinforced by particles that prefer to gather in that interface. These are often carbide particles. When the steel is put into solution that boundary is going nowhere until all of those carbides and particles are gone. And, in order for that grain to grow that boundary has to move. So, if you keep your temperature below that which is required to dissolve those particles, you can’t get grain growth, from normal, or even rather extreme, soaking until those particles are gone. But, if you bump up your temperature to overheating, those particles vanish and you get grain growth in a second. This is why proper soak temperatures are set to pull only the carbon need into solution for proper hardness, while leaving the rest in fine carbide form. This is why .25% V makes W2 a finer grain steel than W1. And this is why soaking does not cause grain growth, and overheating is the actual cause of grain growth regardless of soak time.
Now what about 1095 that has no chromium or vanadium carbides? Won’t soaking grow its grains? Well there are other particles in those boundaries, not as powerful as carbides but better than nothing. There are other particles introduced in the steel manufacturing process that also stabilize the grain boundaries. They may take less time and temperature but they still cause the steel to hold a grain size better. If you want a comparison, use a truly grain size sensitive steel that predates this process. I have made and heat treated my share of ancient type bloomery steel, just iron and carbon, and with it you finally have a steel that you really don’t want to soak, if you can avoid it, as it will begin to grow grains as soon as you reach solution. I normally don't recommend soaks for 10XX series, not due to any real problems, if it is short, but because it is mostly a waste of time.
Edited to add- the importance of soak time is made clearer the closer you are to the critical temperature of Ac1. Decalescence is an endothermic reaction, meaning that it sucks up more heat for itself than the steel normally would need to get hot. This means the higher the heat you go to above it, the more you can feed its transformation. But if you are hovering close to, or within, that transformation zone, decalescence will struggle for every degree it can use and the time to complete is going to grow markedly. Without the temperature, there needs to be time or the transformation will not be complete. One of the best explanations of this can be found in "Tool Steels Simplified" by Palmer and Luerssen.

It is very important to note that this familiar chart is often referred to as the iron carbon equilibrium diagram. This is because the phases shown at the given temperatures are what is achieved at near equilibrium. In plainer English, the phases represented are what you will get if the steel is held at that temperature. It assumes some level of soaking. And the reason the “critical temperature” on heating (Ac1) is very different than the “critical temperature” on cooling (Ar1) is that rising or falling temperatures are no longer holding that equilibrium and pushes the temperatures all over the place. Things dissolve as much as 100°F higher than they come back out of solution, depending on how fast the heating or cooling is. To get them closer together, you need to hold for equilibrium conditions.
Folks can give this a try. Heat a piece of steel until you chase the shadow out of its glow and the magnet stops sticking, i.e. decalescence. Look at how bright this is and remember it. As it cools watch how dim, or dark, it is before you get the brighter energy wave that moves through it and the magnet starts to grab. The steel is almost black again. This is the difference heat and cooling, not holding for a time at a transformation temperature, makes. A1 is but one of four “critical temperatures” we typically deal with in heat treating a knife.

I have shared this image many times before across the internet, but I find that after all these years it is often still needed. This is the fractured end grain size of O-1 that was soaked for 5 hours at around ten degrees higher than its recommended range. I think we can take grain enlargement right off the table for things to worry about with soaking an alloy steel. I have often called grain growth the bladesmith’s bogeyman due to the greatly overexaggerated threat it poses.
What defines an austenite grain is the grain boundary. It is mostly the interface of two different crystalline stacking orientations, but its existence is also reinforced by particles that prefer to gather in that interface. These are often carbide particles. When the steel is put into solution that boundary is going nowhere until all of those carbides and particles are gone. And, in order for that grain to grow that boundary has to move. So, if you keep your temperature below that which is required to dissolve those particles, you can’t get grain growth, from normal, or even rather extreme, soaking until those particles are gone. But, if you bump up your temperature to overheating, those particles vanish and you get grain growth in a second. This is why proper soak temperatures are set to pull only the carbon need into solution for proper hardness, while leaving the rest in fine carbide form. This is why .25% V makes W2 a finer grain steel than W1. And this is why soaking does not cause grain growth, and overheating is the actual cause of grain growth regardless of soak time.
Now what about 1095 that has no chromium or vanadium carbides? Won’t soaking grow its grains? Well there are other particles in those boundaries, not as powerful as carbides but better than nothing. There are other particles introduced in the steel manufacturing process that also stabilize the grain boundaries. They may take less time and temperature but they still cause the steel to hold a grain size better. If you want a comparison, use a truly grain size sensitive steel that predates this process. I have made and heat treated my share of ancient type bloomery steel, just iron and carbon, and with it you finally have a steel that you really don’t want to soak, if you can avoid it, as it will begin to grow grains as soon as you reach solution. I normally don't recommend soaks for 10XX series, not due to any real problems, if it is short, but because it is mostly a waste of time.
Edited to add- the importance of soak time is made clearer the closer you are to the critical temperature of Ac1. Decalescence is an endothermic reaction, meaning that it sucks up more heat for itself than the steel normally would need to get hot. This means the higher the heat you go to above it, the more you can feed its transformation. But if you are hovering close to, or within, that transformation zone, decalescence will struggle for every degree it can use and the time to complete is going to grow markedly. Without the temperature, there needs to be time or the transformation will not be complete. One of the best explanations of this can be found in "Tool Steels Simplified" by Palmer and Luerssen.
Last edited:
J. Doyle
Dealer - Purveyor
Kevin, let me be the first here to say thank you for your tireless efforts to study and understand the methods and processes of our craft and present facts backed up by first hand precise scientific research and your efforts to educate and inform the knifemaking community.
It is very much appreciated and probably doesn't get said enough.
It is very much appreciated and probably doesn't get said enough.
Kevin R. Cashen
Super Moderator
John, no thanks are necessary. Karen just showed me an exchange on social media that I will never visit because I find it toxic, and it reminded me how much I don’t miss such interaction and cringe when my name is involved in its ugliness; I always want to be part of the solution, not part of the problem.
Early in my bladesmithing career I got excited by the things I saw and wanted to share that excitement with others. I remember teaching people how to edge pack, and other things, because I could actually demonstrate it working before my very eyes. In the span of years since, I have realized that our eyes are not to be trusted without a never-ending pursuit of what the actual cause and effects are of what we are witnessing. Much of the information that I share is to make amends for what I could have done to the craft with my hasty conclusions, back then, or even yesterday, if I forget these painful truths. There are no simple answers and, for all its simple appearance, steel can be more complex and fantastic that we can imagine. But its true beauty is that it just is. It doesn’t care about our passions or human disagreements, so why should we?
I have found a very peaceful place, far removed from internet discussions, where I can just listen to steel and learn. If I can share some of what I learn and leave my craft more empowered than before, that is enough. It is only for things that truly worry me that I will do more, but I no longer get the same pleasure from the internet thing that the rest of the population does. And I now have a better ability to sort out those harmless myths and letting them be, to add some quirky interest to the knife scene, and when I see that folks may be going off the road by reading the map wrong I am willing to help.
Early in my bladesmithing career I got excited by the things I saw and wanted to share that excitement with others. I remember teaching people how to edge pack, and other things, because I could actually demonstrate it working before my very eyes. In the span of years since, I have realized that our eyes are not to be trusted without a never-ending pursuit of what the actual cause and effects are of what we are witnessing. Much of the information that I share is to make amends for what I could have done to the craft with my hasty conclusions, back then, or even yesterday, if I forget these painful truths. There are no simple answers and, for all its simple appearance, steel can be more complex and fantastic that we can imagine. But its true beauty is that it just is. It doesn’t care about our passions or human disagreements, so why should we?
I have found a very peaceful place, far removed from internet discussions, where I can just listen to steel and learn. If I can share some of what I learn and leave my craft more empowered than before, that is enough. It is only for things that truly worry me that I will do more, but I no longer get the same pleasure from the internet thing that the rest of the population does. And I now have a better ability to sort out those harmless myths and letting them be, to add some quirky interest to the knife scene, and when I see that folks may be going off the road by reading the map wrong I am willing to help.
EdCaffreyMS
"The Montana Bladesmith"
OK, I'm going to attempt to clear this up. Kevin and I are not arguing! We are speaking the same language, but I think I failed miserably to make myself clear.
My "spunky" input comes from repeatedly having individuals who take what is said/written about soaking to extremes. To explain further, and plainly, I'm referring to those individuals who only grab onto the work "soak" and either fail to read or understand the rest..... then for whatever reason take the attitude... "if some is good then way more, must be way better." They soak blade steels for absurdly long periods...... as in 30, 50, and in many cases beyond an hour.... yes, an hour or more.
Without naming anyone, an example is a young maker who called me recently, asking for my help, and after a LONG conversation, and going through his process step by step, I discovered that he had soaked his 1084 blade for 1 1/2 hours! When I questioned this, he seemed puzzled, and stated that he had found the information of soaking at a specific place on the net, and thought it would be better to do it longer. It gets exasperating dealing with that time and again. I've dealt with emails and phone calls on the subject so often, that I tend to go too far the other direction, when people are soaking steels for those absurdly long time periods. For that I apologize, and in the future will try to restrain myself.
My "spunky" input comes from repeatedly having individuals who take what is said/written about soaking to extremes. To explain further, and plainly, I'm referring to those individuals who only grab onto the work "soak" and either fail to read or understand the rest..... then for whatever reason take the attitude... "if some is good then way more, must be way better." They soak blade steels for absurdly long periods...... as in 30, 50, and in many cases beyond an hour.... yes, an hour or more.
Without naming anyone, an example is a young maker who called me recently, asking for my help, and after a LONG conversation, and going through his process step by step, I discovered that he had soaked his 1084 blade for 1 1/2 hours! When I questioned this, he seemed puzzled, and stated that he had found the information of soaking at a specific place on the net, and thought it would be better to do it longer. It gets exasperating dealing with that time and again. I've dealt with emails and phone calls on the subject so often, that I tend to go too far the other direction, when people are soaking steels for those absurdly long time periods. For that I apologize, and in the future will try to restrain myself.
Kevin R. Cashen
Super Moderator
If there are any more questions that I could help with I would love to discuss them. But let me take time to emphasize that Ed is also pointing out detrimental soak times, which I find just as crazy as he does. If you actually are soaking 1095 for 30 minutes, I have no idea what you are thinking! Industry actually lays it out quite well when they say “one hour per inch of thickness”. The math is pretty simple there. Your average knife blade is around a quarter inch or less… 1 hour divided by four, or less, gives the maximum of around 15 minutes, or less, for a steel that even requires a soak.
Even if you get no grain growth there are still detrimental effects of oversoaking, retained austenite and excess decarb are just two I can think of off the top of my head. You can get it too hot or take too long in forging, you can over-temper the blade or incorrectly normalize, and there is certainly correct and incorrect soak times.
Even if you get no grain growth there are still detrimental effects of oversoaking, retained austenite and excess decarb are just two I can think of off the top of my head. You can get it too hot or take too long in forging, you can over-temper the blade or incorrectly normalize, and there is certainly correct and incorrect soak times.
Chris Railey
Well-Known Member
Ed and Kevin. If you were going to suggest a source for reliable HT “recipes” like a book or chart or something what would that be? Preferably written in redneck terms...
I also know how the soak times got out of control. It’s as simple as if five minutes is good six is better. It continues until one friend tells another he soaks 1095 for 30 minutes. Bam, there you have it.
I also know how the soak times got out of control. It’s as simple as if five minutes is good six is better. It continues until one friend tells another he soaks 1095 for 30 minutes. Bam, there you have it.
tkroenlein
Well-Known Member
Well, I have a question.
It is perhaps less than easily answered, and might even be a little bit influenced by my opinions based on my limited understanding of the subject.
What should we really expect to get from the steel mills? Should knifemakers, who stake their names and reputations on optimized heat treat, expect to get steel in a condition that is ideal for knives? Is it even possible? Is it feasible?
Isn't the steel condition for a forging bladesmith markedly different than a stock removal maker? Doesn't a forged blade get normalized and annealed every single time to set up the steel for HT? Don't we have to take into account the multiple heat and cool cycles a forged blade undergoes when comparing what a flat bar right off the mill needs to reach it's potential? And can that "potential" match the potential of tested, optimized-for-knives HT schedule by an "in-my-hand, one-at-time" knifemaker?
It is perhaps less than easily answered, and might even be a little bit influenced by my opinions based on my limited understanding of the subject.
What should we really expect to get from the steel mills? Should knifemakers, who stake their names and reputations on optimized heat treat, expect to get steel in a condition that is ideal for knives? Is it even possible? Is it feasible?
Isn't the steel condition for a forging bladesmith markedly different than a stock removal maker? Doesn't a forged blade get normalized and annealed every single time to set up the steel for HT? Don't we have to take into account the multiple heat and cool cycles a forged blade undergoes when comparing what a flat bar right off the mill needs to reach it's potential? And can that "potential" match the potential of tested, optimized-for-knives HT schedule by an "in-my-hand, one-at-time" knifemaker?
Chris Railey
Well-Known Member
I am not sure I get the question but what we can reasonably expect from a mill (In my limited opinion) is steel with the proper elements in the proper amounts for the steel that is purchased. In other words 1095 that is made to “specs”. The rest is up to us in how we treat the steel to get what we desire out of it. Now our treatment recipe (for lack of a better term) can only be trusted to produce repeatable results if we receive consistent (made to spec) steel from the mill. Otherwise we will be chasing our tails with inconsistent results. If the mill produces “good consistent steel” then I would be happy. Based off of other conversations with KC I expect ordered steel, even that which advertises it in “fully annealed” state to have some internal stresses so I address that early in my process.
Kevin R. Cashen
Super Moderator
Well, I have a question.
It is perhaps less than easily answered, and might even be a little bit influenced by my opinions based on my limited understanding of the subject.
What should we really expect to get from the steel mills? Should knifemakers, who stake their names and reputations on optimized heat treat, expect to get steel in a condition that is ideal for knives? Is it even possible? Is it feasible?
Isn't the steel condition for a forging bladesmith markedly different than a stock removal maker? Doesn't a forged blade get normalized and annealed every single time to set up the steel for HT? Don't we have to take into account the multiple heat and cool cycles a forged blade undergoes when comparing what a flat bar right off the mill needs to reach it's potential? And can that "potential" match the potential of tested, optimized-for-knives HT schedule by an "in-my-hand, one-at-time" knifemaker?
We should expect a chemistry within a range specified for the alloy it is claimed to be, and we can reasonably expect it to be annealed, probably spheroidized, especially if described as such. Not much beyond that.
I personally believe that if we all were more insistent on being provide spec and data sheets with our material, what we could expect could greatly increase, until then, we get whatever we are given and have to just trust that it is what we were told it is. If it is my tool steel stock, or if I am doing research with it, it must come with spec sheets and papers of origin, or I go elsewhere.
As for optimizing our heat treatments, based upon that initial condition- that is sort of tough because the supplier wants to provide you with material that is annealed to provide the easiest of machining, and this is often in direct opposition to what we want for quick and easy heat treating. Easy machining often means coarse and widely separated carbides, which requires more effort with time and temperature to put into solution.
So, in light of this, you are very much correct that, with proper forging methods, a forged blade will be much more responsive to a quick heat treatment than a stock removed blade, unless that blade is also normalized. I have, on the other hand seen forged blades that were even more difficult to put into solution after improperly low forging heats, which only exacerbated the as received condition.
Kevin R. Cashen
Super Moderator
Ed and Kevin. If you were going to suggest a source for reliable HT “recipes” like a book or chart or something what would that be? Preferably written in redneck terms...
The definitive bible in this would be the "ASM Heat Treater's Guide", but a new edition is quite expensive. What you can do is look on your phone app store for a downloadable facsimile. There are a few apps out there like this. I have one on my phone that allows you to search by alloy name and give you the ASM recipe for heat treating it. If you really want the book, look for older editions from the 1980's in used book stores, it is cheaper and has less extra information that you don't need. Do be aware that these recipes are for meant to be adhered to with standard procedures and thus would work better with the "as-received" materials. Bladesmiths do all kinds of goofy things to steel and then get confused when results vary. To this I would say- when in doubt, normalize!
