Yes Ken,it shows the amps that the machine is pulling. The way I use this machine is, I dial in the voltage I want,lower the piece I am anodizing into the solution then turn on the output power. Watch the amperage spike then drop,when it stops dropping then I turn off the output power and remove my piece.
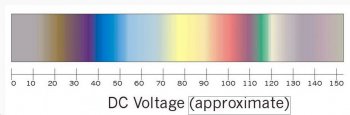
The most common colors I see anodized are bronze,gold and blue. Those colors will all fall below 30 volts. You can go as high as 120 volts with my machine and get many different colors,I think that Michael Walker must have done most of his anodizing in the higher voltages because of the colors that he achieved.
You don't set the amperage on this machine,it shows the amperage that is being drawn and a circut breaker will trip and automaticly reset if too much amperage is drawn,on my machine that is 3 amps. The bigger the surface area of the piece you are anodizing,the higher the amps will be. Like I said,the amperage will spike at first and then gradually drop as the oxidization builds up,when the amps stop dropping your done.