You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Grains, Carbides, and You
- Thread starter me2
- Start date
- Status
- Not open for further replies.
Doug Lester
Well-Known Member
I think that one of the problem areas with carbide growth is slow cooling from critical temperature, as in annealing. I believe it's worse in hypereutectic/hypereuticoid (hell, I can't remember which one Kevin said is correct:20 . Basically, don't do a critical annealing unless you have to soften an air quenching steel. A subcritical annealing, spherodization, will help correct this.
. Basically, don't do a critical annealing unless you have to soften an air quenching steel. A subcritical annealing, spherodization, will help correct this.
Doug
Doug
Kevin R. Cashen
Super Moderator
Let’s try to sort this out. As me2 stated so well, steel is a mixture of carbon and iron. On a whole it is not a compound, it is just a mixture. Because iron is put together in a crystalline way via metallic bonding it does not have molecules, only atoms stacked in an orderly repeating way, this is why you must be very careful mentioning “molecules” in steel since most often you will be wrong. The exception is carbide which is a chemical compound and the two work differently within the same material we call steel.
Let’s deal with the grains first. In metallurgy “grains” is another word for crystal, but it is also more specifically a unit with a single crystalline orientation. As is illustrated here:
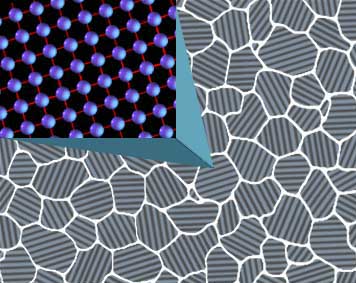
The term “grain direction of the steel” is colloquial nonsense not used in metallurgy, that would be the anisotropic condition and since it is not affected by heat treatment I will not discuss it here. At room temperature the atoms within the crystalline lattice that defines a single grain are stacked in a body centered cubic (bcc) configuration. Bcc does not have many spaces for smaller carbon atoms to fit between the iron atoms so the mixture is very segregated into large iron fields with pockets of carbon in them. But when we heat the iron atoms to 1335F they shift to a new stacking that has lots of spaces for carbon atoms to fit between the iron atoms and we form a more uniform mixture called a solid solution.

This is important because this solid solution is called austenite and it is austenite grains that we refer to whenever we are discussing “grains”. At 1335F when the atoms make the shift to face centered cubic (fcc) they quickly start taking carbon atoms between them to form austenite. New baby grains will form at the corners of the old grain edges and as they dissolve more carbon they will grow to replace the old grains entirely. Once the new grains have replaced the old there will be an equilibrium for a time but with added heat the new grains will then begin to eat each other and continue getting even larger, this is grain growth. Grains reform around the 1335F range (in a simple iron/carbon system) so that defines the temperatures we use for simple grain refinement, which is the easiest and most common occurrence in heated steel. We do it every time we heat the steel to glowing, so it is no big feat and is actually the most basic skill we can master. If you have large grains simply reheat it, with better control this time, and you get all new ones.
Carbides are different, they are the carbon atoms that are not in solution and have gathered in a great enough concentration to chemically bond with the iron (or other metallic elements that may be present); for example simple iron carbide, known as cementite, is Fe3C. These carbides rest outside the iron and iron-carbon solution, and prefer places like the grain boundaries. When we anneal the steel we grow larger carbides by taking as much carbon out of solution as possible to bond in the carbide and leaving soft unfortified fields of iron. When we reheat to make the solution of austenite we dissolve these carbides and put them into the solid solution mixture:
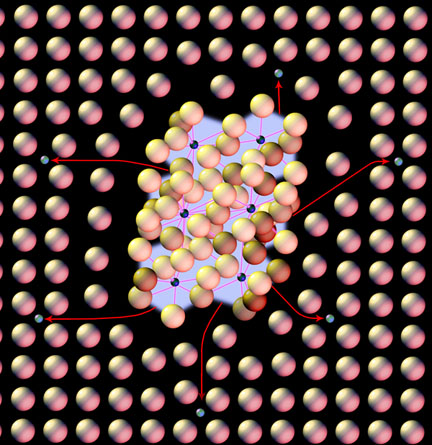
Since carbides are chemically bonded they do not break up and move as easy as the free carbon atoms so we need to throw more heat at them to dissolve them and disperse their carbon evenly, that is why we have soak times and higher temperatures for alloyed steels. When we have things like chrome or vanadium the bonds are very tight and much greater heat is involved in breaking the carbides and that is why you will see temps in excess of 1800F for more richly alloyed steels. If you were to add .5 or .6 percent vanadium to 1084 you could easily reduce its maximum hardness to that of 1040 steel because all of its carbon would be locked in very tough carbides with none left over for proper hardening without extreme overheating.
The other thing to remember about carbide is that it is extremely hard, much harder than the surrounding steel, and this makes it brittle. So having large clusters of carbide is seldom a good thing. It makes machining impossible and can lead to overall embrittlement. Since it is so much harder to abrade than the surrounding material knife edges can only get as fine as the carbides dictate as they will either resist sharpening efforts or pull out and continuously leave large voids in the edge. You can wear away a portion of a grain but no so with a carbide. The worst place to have carbide gather en mass is in the grain boundaries. The preferred path of travel for fracture is often the grain boundaries and if you fill them with the most brittle component of steel you have a weak blade even if it is relatively soft. The ideal carbide condition is as fine as possible and spread and evenly as possible throughout the grains.
How do we control this? It is actually quite easy once we understand the temperature-diffusion relationships. My image of the 1095 with the with the with grain boundaries that me2 linked to is bad but something I did intentionally. I regularly use carbide to highlight grain boundaries when doing metallography. I have had visitors to the shop scratch their heads and ask “you mean you can put carbides exactly where you want inside the steel?” Yes I can, and so can you!
The trick with carbide is tow things, how hot you get it and how fast you cool it. Going hotter dissolves carbides and puts them into solution. Cooling slowly makes carbides, and the slower you cool the larger they get. But be careful since where they will want to grow is seldom what you want, e.g. the grain boundaries. This is why you should avoid slow cooling steels with more than .85% carbon from above “critical”; air cooling is as slow as you want to go. A cooling forge, or a bucket of vermiculite is no place for a hot piece of 1095 or W2!
Proper normalizing, 1600F+, dissolves carbides and even grows grain, but the air cooling leaves you with finer carbides and uniform grain size. Lower temperature thermal cycles will then refine the grain size.
I am sorry if I seem to be repeating me2’s excellent description but I though a reiteration may help people understand better,
Let’s deal with the grains first. In metallurgy “grains” is another word for crystal, but it is also more specifically a unit with a single crystalline orientation. As is illustrated here:

The term “grain direction of the steel” is colloquial nonsense not used in metallurgy, that would be the anisotropic condition and since it is not affected by heat treatment I will not discuss it here. At room temperature the atoms within the crystalline lattice that defines a single grain are stacked in a body centered cubic (bcc) configuration. Bcc does not have many spaces for smaller carbon atoms to fit between the iron atoms so the mixture is very segregated into large iron fields with pockets of carbon in them. But when we heat the iron atoms to 1335F they shift to a new stacking that has lots of spaces for carbon atoms to fit between the iron atoms and we form a more uniform mixture called a solid solution.

This is important because this solid solution is called austenite and it is austenite grains that we refer to whenever we are discussing “grains”. At 1335F when the atoms make the shift to face centered cubic (fcc) they quickly start taking carbon atoms between them to form austenite. New baby grains will form at the corners of the old grain edges and as they dissolve more carbon they will grow to replace the old grains entirely. Once the new grains have replaced the old there will be an equilibrium for a time but with added heat the new grains will then begin to eat each other and continue getting even larger, this is grain growth. Grains reform around the 1335F range (in a simple iron/carbon system) so that defines the temperatures we use for simple grain refinement, which is the easiest and most common occurrence in heated steel. We do it every time we heat the steel to glowing, so it is no big feat and is actually the most basic skill we can master. If you have large grains simply reheat it, with better control this time, and you get all new ones.
Carbides are different, they are the carbon atoms that are not in solution and have gathered in a great enough concentration to chemically bond with the iron (or other metallic elements that may be present); for example simple iron carbide, known as cementite, is Fe3C. These carbides rest outside the iron and iron-carbon solution, and prefer places like the grain boundaries. When we anneal the steel we grow larger carbides by taking as much carbon out of solution as possible to bond in the carbide and leaving soft unfortified fields of iron. When we reheat to make the solution of austenite we dissolve these carbides and put them into the solid solution mixture:

Since carbides are chemically bonded they do not break up and move as easy as the free carbon atoms so we need to throw more heat at them to dissolve them and disperse their carbon evenly, that is why we have soak times and higher temperatures for alloyed steels. When we have things like chrome or vanadium the bonds are very tight and much greater heat is involved in breaking the carbides and that is why you will see temps in excess of 1800F for more richly alloyed steels. If you were to add .5 or .6 percent vanadium to 1084 you could easily reduce its maximum hardness to that of 1040 steel because all of its carbon would be locked in very tough carbides with none left over for proper hardening without extreme overheating.
The other thing to remember about carbide is that it is extremely hard, much harder than the surrounding steel, and this makes it brittle. So having large clusters of carbide is seldom a good thing. It makes machining impossible and can lead to overall embrittlement. Since it is so much harder to abrade than the surrounding material knife edges can only get as fine as the carbides dictate as they will either resist sharpening efforts or pull out and continuously leave large voids in the edge. You can wear away a portion of a grain but no so with a carbide. The worst place to have carbide gather en mass is in the grain boundaries. The preferred path of travel for fracture is often the grain boundaries and if you fill them with the most brittle component of steel you have a weak blade even if it is relatively soft. The ideal carbide condition is as fine as possible and spread and evenly as possible throughout the grains.
How do we control this? It is actually quite easy once we understand the temperature-diffusion relationships. My image of the 1095 with the with the with grain boundaries that me2 linked to is bad but something I did intentionally. I regularly use carbide to highlight grain boundaries when doing metallography. I have had visitors to the shop scratch their heads and ask “you mean you can put carbides exactly where you want inside the steel?” Yes I can, and so can you!
The trick with carbide is tow things, how hot you get it and how fast you cool it. Going hotter dissolves carbides and puts them into solution. Cooling slowly makes carbides, and the slower you cool the larger they get. But be careful since where they will want to grow is seldom what you want, e.g. the grain boundaries. This is why you should avoid slow cooling steels with more than .85% carbon from above “critical”; air cooling is as slow as you want to go. A cooling forge, or a bucket of vermiculite is no place for a hot piece of 1095 or W2!
Proper normalizing, 1600F+, dissolves carbides and even grows grain, but the air cooling leaves you with finer carbides and uniform grain size. Lower temperature thermal cycles will then refine the grain size.
I am sorry if I seem to be repeating me2’s excellent description but I though a reiteration may help people understand better,
Doug Lester
Well-Known Member
Thanks, Kevin, for filling in some blank spaces about carbides.
Just to make sure that I have it right the anisotropic condition mentioned above is the realignment of the iron crystals caused by rolling out the steel at the foundry? Also, would I be correct in saying that pearlite then would be molecules of cementite arranged between "plates" of iron crystals in the form of ferrite? Do other carbides, when present, also form between these plates of ferrite or do those metallic carbides only form outside of the pearlite in hypereuticoid steels when it is cooled slowly enough to cross the pearlite/bainite finish line before falling below the Ms point.
The reason that I'm asking is that I'm trying to understand why with hypoeuticoid alloys like 6150 or S5 that they require a soak at temperature to put the carbon into solution in austinite. Would that be due to the carbides of the tungsten, vanadium, and molybdenum in the alloys and do those carbides exist within the pearlite structure?
Doug
Just to make sure that I have it right the anisotropic condition mentioned above is the realignment of the iron crystals caused by rolling out the steel at the foundry? Also, would I be correct in saying that pearlite then would be molecules of cementite arranged between "plates" of iron crystals in the form of ferrite? Do other carbides, when present, also form between these plates of ferrite or do those metallic carbides only form outside of the pearlite in hypereuticoid steels when it is cooled slowly enough to cross the pearlite/bainite finish line before falling below the Ms point.
The reason that I'm asking is that I'm trying to understand why with hypoeuticoid alloys like 6150 or S5 that they require a soak at temperature to put the carbon into solution in austinite. Would that be due to the carbides of the tungsten, vanadium, and molybdenum in the alloys and do those carbides exist within the pearlite structure?
Doug
One
Banned
Kevin uses big words. What is colloquial nonsense?
"Conversational" nonsense, apparently, in Kevin's opinion. Sorry, but I find that a bit condescending,... especially since anisotropic grain refinement can have an effect on heat treating.
... a topic that get's swept under the rug too often, IMO. Is it “taboo” or something?
One
Banned
Just to make sure that I have it right the anisotropic condition mentioned above is the realignment of the iron crystals caused by rolling out the steel at the foundry? Doug
Doug, it's not a realignment of the crystals. It's created by flaws, porosity and inclusions in the ingot that get stretched out directionally during the rolling process and it's degree of refinement is directly related to “steel quality“. This grain can not be created by heat treatment or altered to any significant degree by heat treatment outside of melting the steel. However, the “quality” of the steel can have an effect on heat treating.
The higher the quality of the steel, the better in responds to heat treating.
Last edited:
Doug Lester
Well-Known Member
So, the alignment of the crystals created as they form as the iron/steel cools from the liquid state is not changed by rolling. It's just the slag inclusions, gas bubbles, etc.
Doug
Doug
One
Banned
So, the alignment of the crystals created as they form as the iron/steel cools from the liquid state is not changed by rolling. It's just the slag inclusions, gas bubbles, etc.
Doug
It does get a bit confusing here again, but I'll try to explain it as simply as I can,… at least how I understand it anyway.
The crystals do get stretched out and realigned, but not permanently. When the crystals get smooshed during hot rolling or forging, as they re-crystallize they form smaller crystals that aren‘t smooshed.
(With cold forging there is no re-crystallization and so the crystals remain deformed. This condition results in “work hardening“.)
What happens with the flaws, inclusions etc., during rolling and forging is a permanent structure in the steel,... and can only be further manipulated through forging...
Hope that helps....
Last edited:
Doug Lester
Well-Known Member
Ok, I'm not a moderator here and I may be overstepping my bounds but I do find the conflict between the art and the science of heat treating and metallurgy a little distressing. Does the discussion of theories/beliefs/practices/industrial standards have to have the flavor of discussing religious writ? We are not discussing the nature of Christ or if the Archangel Gabriel really dictated the Koran to Mohammad. Admittedly, I'm in the scientific camp and I'm not going to believe in things like edge packing anymore than I'm going to believe in Arianism, regardless of who says that it exists. If someone believes in that or that steel has a grain analogous to that of wood, fine. Just remember that your truth is someone else's nonsense and visa versa and try not to get so prickly with people who don't agree with your ideas. Learn to agree to disagree without taking it personal.
To clarify, this is not for or against any particular persons, so please don't take it that way. I've just come across this problem once too often so I decided to address it at this time.
Doug
To clarify, this is not for or against any particular persons, so please don't take it that way. I've just come across this problem once too often so I decided to address it at this time.
Doug
Last edited:
Doug Lester
Well-Known Member
Tai, thanks for your reply on what you are meaning with anisotropic grain. I think that's what my texts say. I just can't find where and in what book.
Doug
Doug
Brad Lilly
Moderator and Awards Boss
Well for better or worse I'm a mod and I'm stepping into Kevin's forum (Sorry Kevin but your not on line now). As long as we keep things nice and polite we can agree to disagree. We have a zero drama policy so if any shows up the thread is locked.
Kevin R. Cashen
Super Moderator
Well for better or worse I'm a mod and I'm stepping into Kevin's forum (Sorry Kevin but your not on line now). As long as we keep things nice and polite we can agree to disagree. We have a zero drama policy so if any shows up the thread is locked.
No problem Brad, your help is most appreciated, we are a team here at Knifedogs. I have always found it a distressing statement about human beings that when one removes all the extra input of inflection, tone, facial expression and body language, as one does with words on a monitor, that folks will invariably assume the worst. Let me clarify the “colloquial nonsense” statement-
“colloquial: a : used in or characteristic of familiar and informal conversation; also : unacceptably informal”
“Nonsense: 1 a : words or language having no meaning or conveying no intelligible ideas”
Putting the two together gives us language that is so informal that is has no meaning to the subject at hand. I chose this because while it is vividly descriptive to refer to steel having a grain direction like wood it is not very accurate. Wood does not have crystals that are more accurately referred to as “grain” but steel does, thus the confusion brought about by referring to a wood like grain is so confusing that it goes beyond having no meaning and is actually misleading. Over the years having folks use this analogy in sentences like “forging aligns the steel grain with the edge” has led to all kinds of confusion. Heat treating deals with austenite grains, the actual crystalline structures in steel, not the anisotropic effects of ingot rolling. Metallurgy prefers precise and accurate terms and when it uses “grain” it is in a specific reference to a crystalline structure, in this context using “grain” as in wood like grain, is like me saying that I have a piece of O-1 that is being ornery. I am not being a lout, or an imbecile by saying my steel is ornery, and we all get the idea of what I am saying, but if I was troubleshooting the problem with a metallurgist it would be colloquial nonsense compared to a precise description of the issues. Lighten up folks, and give others the benefit of the doubt.
Doug Lester
Well-Known Member
Tai, the internet is one of the problems. One of it's biggest strengths is that anyone can publish. One of it's greatest weaknesses is that anyone can publish. I like to have something that can back up the statements that I read there. After all, you can find references that state that a bar of steel records every hammer blow applied to it. Actually, I sat by a stock removal man at a knife show who tried to explain to me how forging distorts the grain in the steel and weakens it. He read it on the internet and you know that you can't put anything on the internet that isn't true. (The last part of that statement is mine but I feel it was implied) I've also read that anyone who says that you can forge weld in a gas forge is just blowing smoke at you; that only coal or coke can reach the required temperatures. I have to admit that I, through my ignorance, have occasionally put out some high grade bovine fertilizer on the these boards. A good thing that there are others to review what is said and I appreciate the correction if someone puts forth a better reason than "I know what I'm talking about and, if you disagree with me, you don't". You really have to be able to separate the wheat from the chaff.
Doug
Doug
Last edited:
Kevin R. Cashen
Super Moderator
Thanks, Kevin, for filling in some blank spaces about carbides.
Just to make sure that I have it right the anisotropic condition mentioned above is the realignment of the iron crystals caused by rolling out the steel at the foundry? Also, would I be correct in saying that pearlite then would be molecules of cementite arranged between "plates" of iron crystals in the form of ferrite? Do other carbides, when present, also form between these plates of ferrite or do those metallic carbides only form outside of the pearlite in hypereuticoid steels when it is cooled slowly enough to cross the pearlite/bainite finish line before falling below the Ms point.
The reason that I'm asking is that I'm trying to understand why with hypoeuticoid alloys like 6150 or S5 that they require a soak at temperature to put the carbon into solution in austinite. Would that be due to the carbides of the tungsten, vanadium, and molybdenum in the alloys and do those carbides exist within the pearlite structure?
Doug
Doug, there are many things that happen during the solidification of the steel ingot in traditional steel production. One is the formation of segregated structures such as dendrites as different components come out of solution and solidify at differing rates, the most striking example is seen on the far right of the iron-carbon equilibrium diagram where the iron-carbon eutectic point actually rests (the eutectoid is on the left hand side and is what we are more familiar with). Add to this pipe, blowholes, slag inclusions, scabbing etc… and you can see why we want to roll this stuff and break that crap up a bit. It all gets drawn out in lengthwise in the direction of rolling to make something almost like random damascus out of all such steel, except on such a fine scale that it is rather different. Even so when we cycle steel on the wrong way the alloy segregation can form carbide concentrations that can look very damascus like, you probably have heard it referred to as alloy banding. The actual crystals of the steel do not follow this same paradigm as they are reformed on every heating cycle.
Pearlite is really cool stuff and you pretty much have it correct. When we cool a bar of austenitic steel the carbon comes out of solution at points of higher energy, like ice in a pond forming around a twig or rock candy forming on a string. The ideal spots for this are corners of the grain boundaries, and other inconsistencies in the crystalline array. This is why finer grain has lower hardenability, finer grains equal more gain boundaries and more points for pearlite to start. I have a cool micrograph of a perlite colony that spans two austenite grains because of a carbide in the grain boundary what its point of nucleation. The reaction works like this, a carbide seed begins the cascade as the carbon comes out of the austenite solution. This then forms a lengthwise growing lamellae of cementite (iron carbide). As the carbide lamellae grows it depletes a band of austenite to either side of carbon until it is nothing but ferrite (iron) and this continues with another cementite formation next to them and on and on until the austenite grain is replaced with pearlite within the prior austenite boundaries. This is why austenite grain still define “grains” even when the steel is in another phase, because all these phases form within he austenite framework.
Since alloying beyond carbon is often substitutional atoms (as big or bigger than iron atoms so that they can’t move between them) the other carbides beyond cementite do form as randomly and tend to be less mobile. These you will find scattered in various places throughout. When you slow cool from Arcm to Ar1 (sorry folks I assume Doug will get this and it will take another couple of paragraphs to explain) the carbon coming out of solution will find these substitutional atoms appealing. You will often find these other carbides between martensite needles and they will also grow during many tempering operations where they can be responsible for the differing forms of secondary hardening and tempering embrittlement.
Hypoeutectoid steels often need an extra bit of heat or time simply to distribute the carbon even through all the excess ferrite, but if there are carbide former present than you will need that extra effort to break the carbides and free up the carbon for proper austenite solution. This is where a steel can actually have a carbon content of a hypereutectoid, but then behave as a hypoeutectoid on the iron carbon equilibrium diagram due to having its carbon locked up in carbide.
One
Banned
Tai, the internet is one of the problems. One of it's biggest strengths is that anyone can publish. One of it's greatest weaknesses is that anyone can publish. I like to have something that can back up the statements that I read there. Doug
Very true, and that's why the basics are so important. We can use them to weed out what is gibberish and what isn't.
Doug Lester
Well-Known Member
Thanks, Kevin. I'll have to chew on that for a bit. Could you check the last sentence in your answer and see if you swithched hyper and hypoeuticoid or if it's right as you wrote it? Regardless, as I understand your answer, when pearlite forms it is cementite that plates between the plates of ferrite and if there are other carbides present, they exist outside of the pearlite. Thanks, the books I have weren't clear on this point and a lot of the diagrams just say carbides and let the reader figure out which one's are being talked about.
Doug
Doug
Kevin R. Cashen
Super Moderator
Thanks, Kevin. I'll have to chew on that for a bit. Could you check the last sentence in your answer and see if you swithched hyper and hypoeuticoid or if it's right as you wrote it? Regardless, as I understand your answer, when pearlite forms it is cementite that plates between the plates of ferrite and if there are other carbides present, they exist outside of the pearlite. Thanks, the books I have weren't clear on this point and a lot of the diagrams just say carbides and let the reader figure out which one's are being talked about.
Doug
Nope, it is right and should read- This is where a steel can actually have a carbon content of a hypereutectoid (above .8%), but then behave as a hypoeutectoid (below .8%) on the iron carbon equilibrium diagram due to having its free carbon locked up in carbide.
- Status
- Not open for further replies.